H5N1
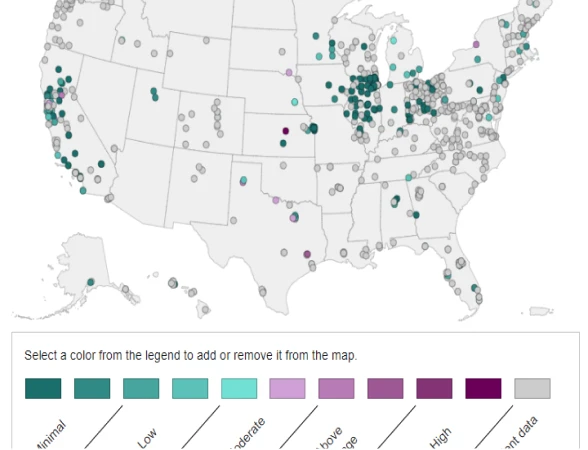
CDC Announces Influenza A Dashboard
Most Requested Kits



(Cat# 100700)
A molecular reagent kit consisting of all primers, probes, and controls necessary for wastewater surveillance of H5* influenza and pan-influenza A. Assay solutions and appropriate controls are provided for the quantification of H5* influenza and pan-influenza A. Through the use of this kit in conjunction with the Bio-Rad QX200™ or QX600™ Droplet Digital™ PCR Systems, users can monitor H5* influenza and pan-influenza A levels. Contains enough material for 200 reactions.
GT-Digital H5* Wastewater Surveillance Assay for the Bio-Rad QX200™ or QX600™ Droplet Digital™ PCR System (Beta)
Molecular reagent kit consisting of all primers, probes, and controls necessary for wastewater surveillance of avian H5* influenza. Assay solutions and appropriate controls are provided for the quantification of avian H5* influenza. Through the use of this kit in conjunction with the Bio-Rad QX200™ or QX600™ Droplet Digital™ PCR System, users can monitor avian H5* influenza levels at the community level.
*Disclaimer! This assay was designed to ensure detection of H5N1 subtypes from avian, cattle, and human origin. Importantly, this assay is predicted to
detect other H5 subtypes of influenza such as H5N5, H5N6, etc. However, this assay is not predicted to cross-react with other subtypes of influenza A
such as H1 and H3.


Featured Testing Service: Influenza H5 Milk Testing
Detect Influenza H5 subtypes in milk, including H5N1. Contact us to learn more or start testing today!
Contact usKey Benefits
Streamlined workflows to differentiate between multiple pathogens. Save time, cost and reagents. Optimized for high output efficiency.
Off-the-shelf validated kit comes with all primers, probes and controls. Easy-to-follow, step-by-step instructions and technical support.
Options available for multiple platforms including digital and RT-PCR. Contact us to see what kits are available for your lab!
No predicted cross-reactivity using our proprietary bioinformatics pipeline. We put in the research to provide the best products available.
GT Molecular’s PCR
Advantages and
Applications